2015/2/4 16:22:07

The state food and drug administration and update the latest production approval of the approval time is on December 25, 2014. New batch of production approval, a total of 478, 2014 (according to accept order record, delete and then register the relevant approval, 418 domestic, import 60). Chemicals in 438 occupies 93% share of the overall approval number, traditional Chinese medicine (TCM) with 18 second, biopharmaceutical 13 production approval third (see chart 1). Chemicals, a domestic manufacturer approval number is 383, imports of 55 pieces.

In medicine of generic terms (does not contain excipients, active pharmaceutical ingredients, intermediates, formulations), ammonia bromine to the maximum number of approved, a total of 10 production approval, followed by sora azole of the seven, the third is the panxi tora of 6 PCS. Ammonium bromide and LAN sola azole are released by the food and drug administration excessive repeated declaration registered drug varieties directory.
There are three common name for his class products, respectively is for and path mesylate imatinib. Mesylate path for piece hengrui filing in 1.1 class new medicine, API mesylate path for approvals obtained by jiangsu sdsensor pharmaceutical company. Shiyao group, the Italian pharmaceutical co., LTD. Of imatinib mesylate tablets is listed on the second. Gleevec in jiangsu, in 2013 the first domestic a generic listing. Newly approved drugs and diabetes related DDP - 4 is novartis metformin d Glenn dean (Ⅱ) and metformin d Glenn dean (Ⅲ), new listing in December 2014. In the digestive system drugs, orsay kang pharmaceutical co., LTD., jiangsu's rob ray Bella azole sodium for injection in December of this year, nanjing long Australian pharmaceutical co., LTD. Enjoy the exclusive of the dosage form period more than half a year.
No Chinese medicine injection approved this year.
From the point of domestic provinces, 77 production approval of Jiangsu province ranked first, accounting for 19% of the companies within the new batch of production approval number, followed by Sichuan and Zhejiang.

As shown in table 1, 2014 has 17 production around December 24, on-site inspection, the examination and approval to complete - DaiZhi certificate ", the shenzhen microchip biotechnology co., LTD. 1.1 new drugs and west to the west of the amine amine piece have attracted the most attention. This product is said to be China's first approved the FDA approved for clinical research in the United States of China chemical original new drugs, has completed the phase I clinical trial research.

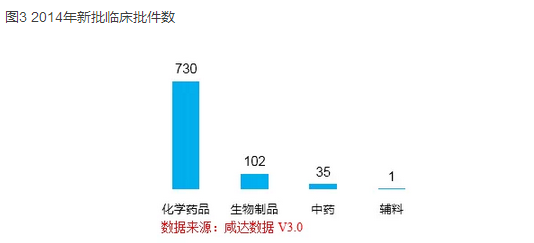
According to the latest statistics of salty V3.0 of data (as of December 31, 2014), the country to update the latest clinical approval granted by the corresponding approval time is on December 25, 2014, to state the start time is "2014", a total of 868 clinical approval documents approved. Row of chemicals in 730 for testing first. Biological products, a total of 102 clinical entity, of which the therapy with 70 other biological products for testing, 32 preventive biological products.
Chemical drugs in clinical batch number, belongs to the class 1.1 68, 3.1 class 242 pieces, 6 class has 55 pieces. 1.1 class new medicine clinical batch number 2014 for most of the enterprise is jiangsu hengrui, eight parts, mainly cefuroxime Glenn, cefuroxime, Craig pan-black slice, ring mi ji and ring mi DE ji piece; Followed by zhejiang sea is (hai bo not Ming, hai bo Ming capsule, HPPH and injected with HPPH), again is jiangsu howson (HS - 10182 and mesylate fluorine for horse pills) and jiangsu sheng (APG - 1387, injection with APG - 1387, R - (-) - acetic acid gossypol and R - (-) - acetic acid gossypol tablets). 3.1 class for testing approved several of the top three basic it is research and development enterprises, respectively is shandong innovative drug research and development co., LTD. (10), nanjing huawei pharmaceutical technology development co., LTD. (9), shandong gonadorelin pharmaceutical technology development co., LTD. (8) and Beijing Fred anzheng pharmaceutical technology research institute (8). Declare 6 class top three, respectively is zhejiang huahai (7) scale drug research institute, Beijing day day scale nanyang pharmaceutical factory (4 pieces) and chongqing medicine pharmaceutical co., LTD. (3).
Single kind of common name to their country's first approved clinical resistance with Janssen Biotech of anthropogenic interleukin 6 monoclonal antibody (CNTO136) injection and injection of choose and employ persons the source resistance IL - 17 a monoclonal antibody (CNTO6785), Shanghai sea of traditional Chinese medicine science and technology development co., LTD of iodine (131 I) love grams of resistance to injection, paekche shenzhou (Beijing) biological technology co., LTD. For injection Intetumumab single resistance (the wood single resistance). Roche's unlisted duly para bead sheet resistance injection (treatment resistance to epidermal growth factor receptor 2, the treatment of HER2) to get approval again, it is worth noting that the health of Shanghai citic countries for injection restructuring Ⅱ type of tumor necrosis factor receptor - antibody fusion protein has previously approved two specifications, the 2014 vial restructuring the HER2 humanized monoclonal antibody and approved clinical. Shenzhou cell engineering of restructuring the anthropogenic resistance to epidermal growth factor receptor monoclonal antibody injection also in 2014 approved by the clinic. Human recombinant - mouse chimeric anti CD20 monoclonal antibody injection in 2014 zhejiang sea, Shanghai forte macro han lam and approved clinical cinda creatures. Shenyang stroke of humanized against people TNF alpha monoclonal antibody injection (CHO cells) and zhuhai injection method with a recombinant humanized anti-tumor necrosis factor alpha monoclonal antibodies are approved for clinical.
For 2014, for the first time in their common name approved for clinical Refametinib, exceed China and maleic acid, ivey, for Bosutinib, Ibrutinib, toluene sulfonic acid Eric for, acyclovir, mesylate for fluorine horse for and afar for such as clinical approval again. 's hangzhou east China pharmaceutical group new drug research institute co., LTD. Of China for pharmaceutical research co., LTD., hangzhou eisen for and maleic acid ivey howson medical group in jiangsu lianyungang macro hit a mesylate fluorine horse for pharmaceutical co., LTD., is 1.1 class declaration. Wallace China for channels of major projects is variety, on March 25, 2014, CDE, July 25, 2014 varieties of major projects began to review, September 30 varieties of major projects completed review, October 31, 2014 for clinical entity.