2015/2/4 16:03:16

FDA approved drugs as fruitful year in which the field of anti-tumor drugs approved nine, became the field to get the most approved drugs. Secondly, four diabetes drugs, four new antimicrobial drugs, including new antibacterial drugs currently approved in this field weak drug development, undoubtedly plays a positive role in promoting.
January
★ Kenny column net
Indications: Type 2 diabetes
Aspect: sodium - glucose cotransporter 2 (SGLT2) inhibitor approved by the FDA Dag column net is second SGLT2 inhibitors, by inhibiting the expression in the kidney of SGLT2, reducing renal glucose reabsorption, thereby lowering plasma glucose levels .
He ★ US Secretary Joan
Indications: 24 hours of non-blind patients waking disorders (Non-24)
Aspect: This is the first indication only drug approved. Non-24 is a serious, rare, circadian rhythm disorders and chronic diseases.
February
★ elosulfase alfa
Indications: mucopolysaccharide deposition disease type IVA
Aspect: the first to be approved by the FDA mucopolysaccharide deposition disease
IVA-type drugs, approved two weeks earlier than expected. Mucopolysaccharidosis type Ⅳ (Morquio syndrome), there are two subtypes: ⅣA galactose 6-sulfatase (GALNS) deficiency, ⅣB for β-D-galactosidase deficiency. The disease is an autosomal recessive inheritance, the clinical features of significant growth retardation, skeletal deformities and gait abnormalities and gradually significantly more patients with life expectancy of 20 to 30 years old.
★ droxidopa
Indications: primary autonomic nervous breakdown (Parkinson's disease, multiple system atrophy and pure autonomic nervous breakdown), dopamine β-hydroxylase deficiency, neurological symptoms in non-diabetic autonomic neuropathy in patients with endogenous orthostatic hypotension (NOH)
Aspect: droxidopa is the first and only drug approved by the FDA for the treatment of NOH, is nearly 20 years NOH symptomatic treatment of the first new treatment options, the FDA granted accelerated approval.
★ metreleptin
Indications: systemic metabolism due to lipodystrophy, can not produce enough leptin caused abnormalities, including insulin resistance
Aspect: metreleptin as an alternative therapy for the disease, which is the first drug treatment for the disease.
March
★ Florbetaben F18
Indications: Alzheimer's disease
Aspect: Florbetaben F18 is the FDA approved the first three Alzheimer's disease diagnostic reagents for PET scans to detect β- amyloid plaques, before also approved Vizamyl
(Flutemetamol F18), Amyvid (florbetapir F18), florbetaben F18 and flutemetamol
F18 belong to the same compound.
★ miltefosine
Indications: leishmaniasis
Aspect: miltefosine is the first FDA-approved treatment of skin or mucous leishmaniasis drugs. Leishmaniasis is a disease caused by Leishmania, spread by the bite of sand flies to humans, the disease occurs mainly in tropical and subtropical regions.
★ Appleton West
Indications: active psoriatic arthritis
Aspect: clinical application Appleton Manchester continues to develop, it may be applied in the future rheumatoid arthritis, Crohn's disease, ulcerative colitis, and other indications of treatment. According to
FDA statement, as a post-listing requirements, manufacturers will be registered through a pregnancy study evaluated the effects of drug exposure in pregnant women.
April
★ A must LUTAI
Indications: Type 2 diabetes
Aspect: A must belong Lutai glucagon-like peptide -1 (GLP-1) receptor agonist. A weekly subcutaneous injections will Lutai, combined with diet and exercise, can improve glycemic control in patients with type 2 diabetes.
★ ramucirumab
Indications: advanced gastric or gastroesophageal junction adenocarcinoma
Aspect: ramucirumab is specifically blocking vascular endothelial growth factor receptor 2 (VEGFR2) and people downstream angiogenesis pathway humanized monoclonal antibodies. ramucirumab been approved for the treatment of chemotherapy failed gastric or gastroesophageal junction adenocarcinoma.
★ siltuximab
Indications: Orphan multicentric Castleman's disease (MCD)
Aspect: The disease known as Castleman's disease (Castleman's disease, CD), one case of unknown cause reactive lymphadenopathy, clinical relatively rare. The disease occurs mainly in adults, due to excessive production of a certain type of white blood cells leading to the lymph nodes, the disease may weaken the immune system, making it difficult to fight infection. Patients usually occurs at night sweats, fever, weight loss and weakness and other symptoms.
★ color Rui imatinib
Indications: Late (transfer) non-small cell lung cancer (NSCLC)
Aspect: color is a Swiss imatinib anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor, blocked the promotion of cancer cell proteins for accepting transfer ALK- positive NSCLC patients after imatinib treatment of gram azole treatment. G azole imatinib was previously only approved ALK tyrosine kinase inhibitor.
May
★ Walla Pasha
Indications: heart disease, stroke and other cardiovascular diseases
Aspect: Walla Pasha is an initiative of the protease-activated receptor 1 (PAR-1) antagonists, can inhibit the formation of clots in blood clotting. PAR-1 is a receptor that can be activated by thrombin, and thrombin is a potent platelet activator. Walla Pasha can inhibit platelet PAR-1 receptor, thereby inhibiting thrombin-induced platelet aggregation.
★ vedolizumab
Indications: moderate to severe Crohn's disease and ulcerative colitis (UC)
Aspect: The medicament is an injectable monoclonal antibodies, suitable for patients who are on one or more conventional treatment (e.g., corticosteroids, immunomodulatory agents, tumor necrosis factor inhibitors) do not answer.
★ dalbavancin
Indications: acute bacterial skin and skin structure infections (ABSSSI)
Aspect: dalbavancin is the first and only intravenous (IV) an approved treatment for ABSSSI double dose regimen of antibiotics. It belongs to the second generation, semi-synthetic glycopeptide fat can be a lipophilic side chains of sugar added to an enhanced peptide backbone. In vitro drug for multiple Gram-positive bacteria (including MRSA and S. pyogenes) and other streptococcal bacteria exhibited bactericidal activity, for the treatment of Gram-positive bacteria (including methicillin-resistant Staphylococcus aureus, MRSA) acute bacterial skin and skin structure infections caused.
June
★ phosphate Thailand to acetazolamide
Indications: acute bacterial skin and skin structure infections (ABSSSIs)
Aspect: phosphate Thailand to acetazolamide specific objectives approved by the Gram-positive bacterial infections, including: Staphylococcus aureus (including methicillin-resistant [MRSA] and methicillin-sensitive [MSSA] strains), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus group angina (including angina streptococci, Streptococcus intermedius and Streptococcus constellations) and Enterococcus faecalis.
★ efinaconazole
Indications: onychomycosis
Aspect: The drug is the first topical antifungal triazole. Onychomycosis is a place in the human finger, commonly known as infectious diseases (toe) on, is called by a large class of microbial infections caused by pathogenic fungi, inadequate treatment of the disease, largely due to the existing treatment limitations of the drug.
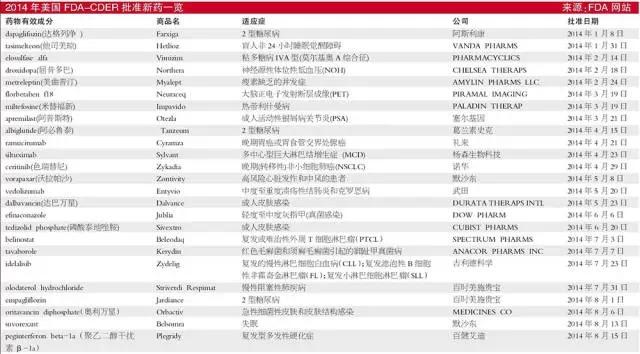
July
★ Belinostat
Indications: aggressive non-Hodgkin's lymphoma (NHL)
Aspect: This is the third since the 2009 NHL approved for the treatment of this rare type of drug. The other two drugs were approved in 2009 Pula folate analogue metabolic inhibitor song sand injection (Folotyn), and in 2011 approved a histone deacetylase (HDAC) inhibitor romidepsin (Istodax). This medicine is applied to at least have received a treatment of PTCL patients.
★ Tavaborole
Indications: toenail fungus infection
Aspect: Tavaborole is the first boron-oxygen for the treatment of toenail infections olane (oxaborole) antifungal. Tavaborole future is also expected for fingernail infections.
★ idelalisib
Indications: relapsed chronic lymphocytic leukemia (CLL), follicular lymphoma (FL) and small lymphocytic lymphoma (SLL)
Aspect: idelalisib oral kinase inhibitor, and rituximab (Rituxan) combination therapy relapsed CLL, as monotherapy in relapsed follicular B-cell FL and recurrent SLL. The FDA granted accelerated approval after two indications requiring systemic therapy received before the patient at least twice.
★ olodaterol
Indications: Chronic obstructive pulmonary disease (COPD)
Aspect: The drug is an inhaled long-acting β- adrenergic agonists (LABA), helps to keep the muscles around the airway relaxation and prevention of disease symptoms.
August
★ empagliflozin
Indications: Type 2 diabetes
Aspect: empagliflozin is a sugar, sodium cotransporter 2 (SGLT2) inhibitor, blocked the renal glucose reabsorption, increased glucose excretion and lower blood sugar levels. Once daily oral administration of the drug.
★ oritavancin
Indications: acute bacterial skin and skin structure infections (ABSSSIs)
Aspect: The drug is FDA-approved antibiotic therapy for ABSSSIs first and only a single dose treatment regimen.
★ suvorexant
Indications: Sleep Disorders
Aspect: The new hypnotic drug suvorexant was first approved orexin receptor antagonist that works by blocking the neuropeptide orexin A and orexin B receptor binding, thereby inhibiting the activation of neurons to wake up the system.
★ pegylated interferon β-1a
Indications: relapsing multiple sclerosis (MS)
Aspect: pegylated interferon β-1a in the interferon β basis through structural improvements, to extend the half-life of the drug, which does not affect the efficacy of the situation and reduce the frequency of administration. Specific dosage regimens of subcutaneous pegylated interferon β-1a, once every two weeks.
★ eliglustat
Indications: Type 1 Gaucher disease
Aspect: the drug for long-term treatment of type 1 Gaucher disease in adult patients.
September
★ pembrolizumab
Indications: advanced or unresectable melanoma
Aspect: pembrolizumab is the first FDA-approved only people programmed death receptor -1 (PD-1) class of drugs.
★ naloxegol
Indications: opioid-induced constipation
Aspect: naloxegol are acting on the outer periphery of the opioid receptor antagonist, used to alleviate cancer pain in adult patients with chronic non-opioid constipation affected.
★ dulaglutide
Indications: Type 2 diabetes
Aspect: The drug is subcutaneous injection once a week, is a glucagon-like peptide -1 (GLP-1) receptor agonist.
October
★ sofosbuvir / ledipasvir
Indications: genotype 1 hepatitis C (HCV) infection
Aspect: The compound preparation named Harvoni. It was the first approved for the treatment of genotype I HCV infection, and does not require a full oral combined injection drug interferon or ribavirin anti-hepatitis C program.
★ netupitant / palonosetron hydrochloride
Indications: patients with cancer chemotherapy nausea and vomiting
Aspect: its compound preparation named Akynzeo. palonosetron hydrochloride was approved in 2008 for the prevention of nausea and vomiting after cancer chemotherapy began the acute phase (within 24 hours) generated. netupitant is a new drug for the prevention of cancer chemotherapy after the start of acute and delayed phase (from 25 to 120 hours after chemotherapy), nausea and vomiting.
★ sulfur hexafluoride lipid-type a microspheres
Indications: echocardiogram left ventricular cloudy
Aspect: This medicine is used echocardiographic images illegible, improve the left ventricular endocardial border demarcation.
★ nintedanib and pirfenidone
Indications: Idiopathic pulmonary fibrosis
Aspect: Both new drugs have received FDA approval quickly, priority review, four kinds of breakthrough drugs and orphan drug status.
December
★ blinatumomab
Indications: Before chromosome-negative B-cell acute lymphoblastic leukemia (B- cell ALL)
Aspect: immunotherapy has a unique mechanism of action, especially blinatumomab, leukemia patients for better effect. FDA has taken the initiative in consultation with the sponsor to make a breakthrough drug therapies designated to promote the ratification of the new drugs.
★ finafloxacin
Indications: Acute otitis externa
Aspect: finafloxacin belong to the latest FDA-approved fluoroquinolone antimicrobial drugs for the treatment of acute otitis externa by Pseudomonas aeruginosa and Staphylococcus aureus caused.
★ ceftolozane / tazobactam
Indications: complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI)
Aspect: ceftolozane containing cephalosporin antimicrobial drugs ceftolozane and β- lactamase inhibitor tazobactam combination products.
★ olaparib
Indications: Late defective BRCA gene therapy of ovarian cancer
Aspect: olaparib is poly ADP- ribose polymerase (PARP) inhibitor, to participate in repairing damaged DNA. Approved olaparib while, FDA also approved the companion diagnostic test products BRACAnalysis
CDx.
★ ombitasvir / paritaprevir / dasabuvir
Indications: genotype 1 chronic hepatitis C virus (HCV) infection, including advanced cirrhosis
Aspect: This combination product called Viekira
Pak, three new drugs ombitasvir it contains, paritaprevir and dasabuvir synergistic inhibition of HCV growth. Also includes previously approved drug ritonavir, used to increase blood levels of paritaprevir.
★ peramivir
Indications: influenza infection
Aspect: peramivir influenza virus neuraminidase inhibitors, neuraminidase is virus particles from infected cells release enzymes. Neuraminidase inhibitors are commonly used for treating influenza infections. Peramivir is approved only for the first neuraminidase inhibitor, intravenous (IV) administration.
★ nivolumab
Indications: advanced melanoma
Aspect: nivolumab is outside pembrolizumab, another year, the only FDA accelerated approval PD-1 drug for other drugs ineffective for not resectable or metastatic (advanced) melanoma treatment.